天然产物全合成研究组 天然产物全合成研究组
|
Home Research Ang Li Members Publications News Photos Join us |
|---|---|
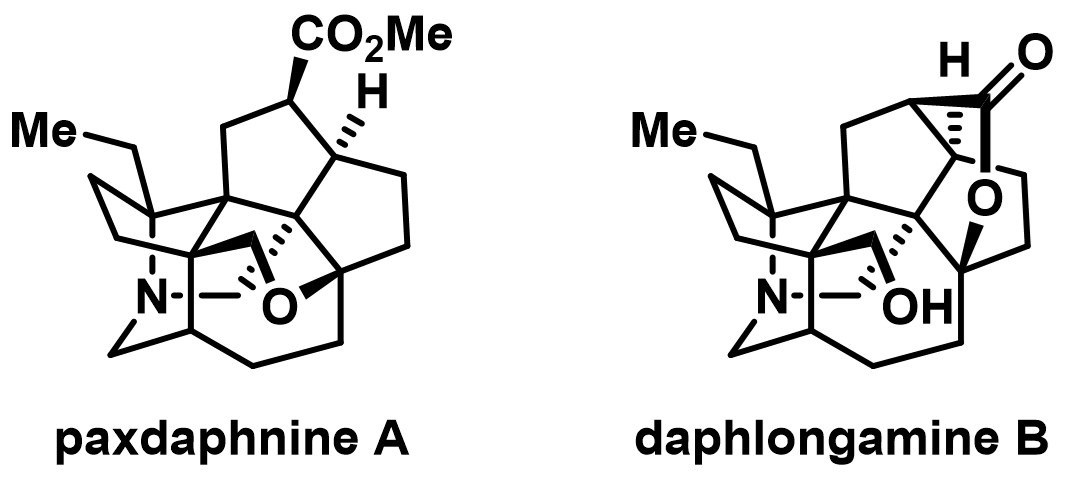
Publications 80. Lu Ren, Zhaohong Lu, Dimin Wu, Mingchen Ma, and Ang Li,*Total synthesis of paxdaphnine A and daphlongamine B via an aza-prins cyclization strategy, J. Am. Chem. Soc. 2025, 147, 27137–27142 (L. R., Z. L. and D. W. contributed equally).
79. Jiulong Li, Pengxin Ren, Lin Wang, Yuting Zhang, Peng Yang,* Weiwei He,* and Ang Li,* Inverse-electron-demand Diels–Alder approach to densely substituted bicyclo[3.2.2]nonanes: Discovery of an autophagy regulator, Chin. Chem. Lett. DOI:10.1016/j.cclet.2025111381.
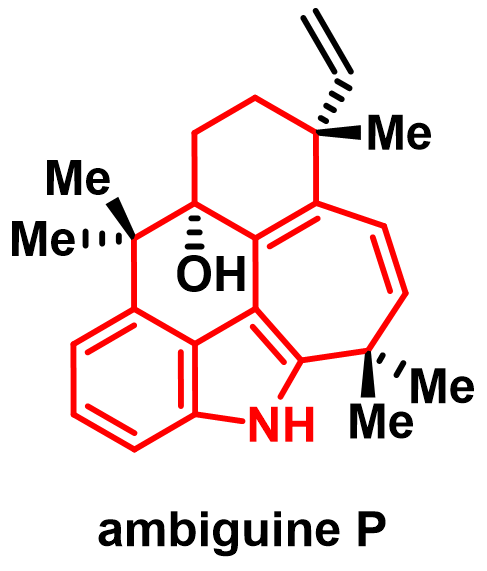
78. Yifan Fei, Bo Fan, Zhigang Liu, Mengyu Ba, Zhongwen Cui, Peng Yang,* and Ang Li,* Concise total synthesis of ambiguine P, J. Am. Chem. Soc. 2025, 147, 18391–18396 (Y. F., B. F. and Z. L. contributed equally).
77. Yali Wu, Shaonan Wang, Zhicong Guo, Min Sun, Zhen Xu, Yu Du, Fahui Zhu, Yajuan Su, Zhou Xu, Yi Xu, Xu Gong, Ruan Fang, Jiaojiao Hu, Yan Peng, Zhaowen Ding, Cong Liu, Ang Li,* and Weiwei He,* Hapalindole Q suppresses autophagosome-lysosome fusion by promoting YAP1 degradation via chaperon-mediated autophagy, Proc. Natl. Acad. Sci. U.S.A. 2024, 121, e2400809121 (Y. W., S. W., Z. G. and M. S. contributed equally).
76. Kaien Liu, Youqi Tao, Qinyue Zhao, Wencheng Xia, Xiang Li, Shenqing Zhang, Yuxuan Yao, Huaijiang Xiang, Chao Han, Li Tan, Bo Sun, Dan Li, Ang Li, and Cong Liu,* Binding adaptability of chemical ligands to polymorphic α-synuclein amyloid fibrils, Proc. Natl. Acad. Sci. U.S.A. 2024, 121, e2321633121 (K. L. and Y. T. contributed equally).
75. Dongping Chen, Xiang Zhang, Anastassia Andreevna Vorobieva, Ryo Tachibana, Alina Stein, Roman P. Jakob, Zhi Zou, Damian Alexander Graf, Ang Li, Timm Maier, Bruno E. Correia,* and Thomas R. Ward,* An evolved artificial radical cyclase enables the construction of bicyclic terpenoid scaffolds via an H-atom transfer pathway, Nat. Chem. 2024, 16, 1656–1664.
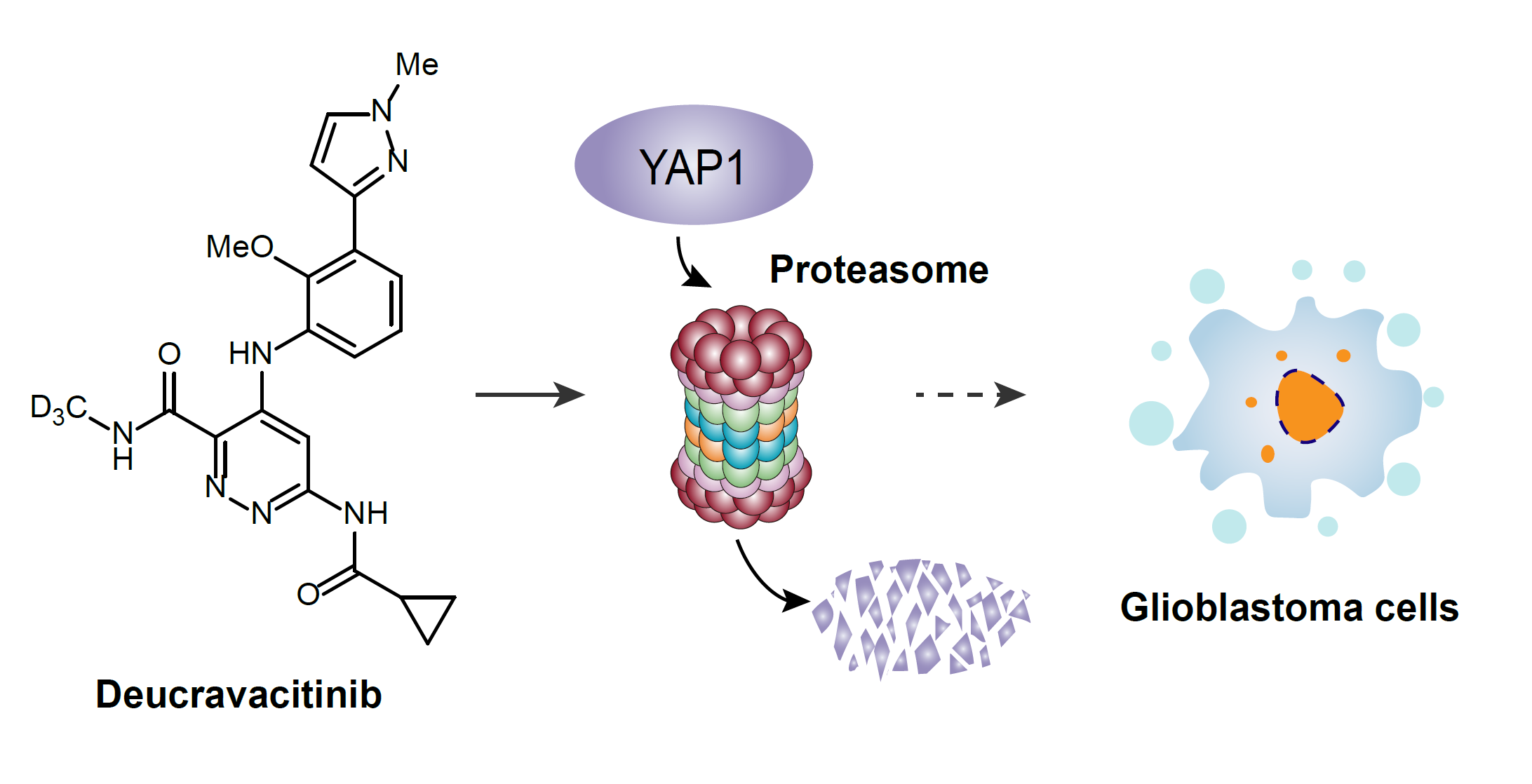
74. Shaonan Wang, Min Sun, Yajuan Su, Zhou Xu, Ang Li,* and Weiwei He,* Deucravacitinib induces proteasomal degradation of YAP1 in human glioblastoma cells, Synlett. DOI: 10.1055/a-2331-6463.
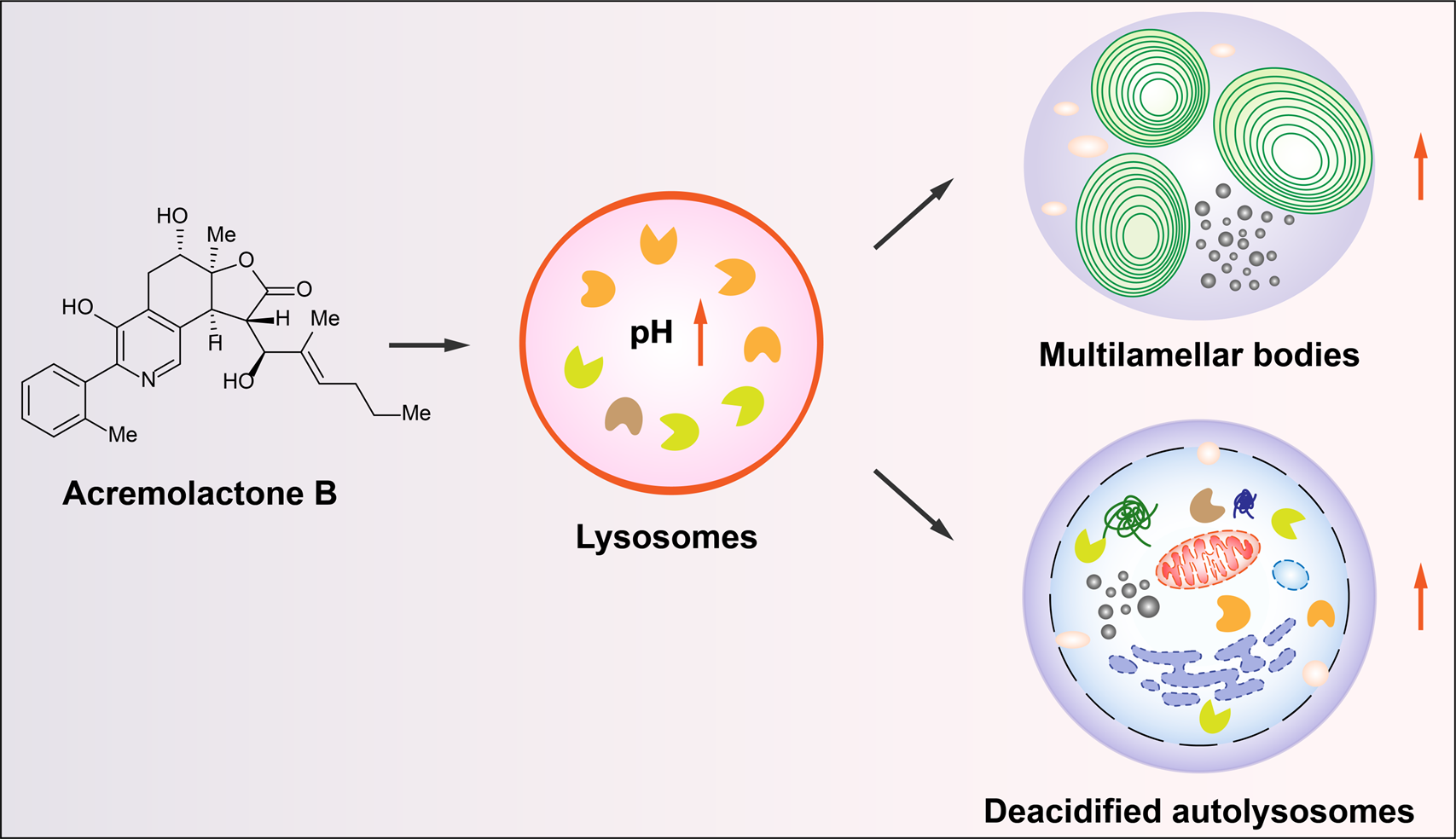
73. Shaonan Wang, Yali Wu, Mengyu Ba, Zhou Xu, Guoxing Gu, William G. Whittingham, Cong Liu, Ang Li,* and Weiwei He,* Discovery of the lysosome-inhibiting function of acremolactone B, Synlett. DOI: 10.1055/a-2325-3938.
72. Mengyu Ba, Fengqi He, Lu Ren, William G. Whittingham, Peng Yang, and Ang Li,* Scalable total synthesis of acremolactone B, Angew. Chem. Int. Ed. 2024, 63, e202314800.
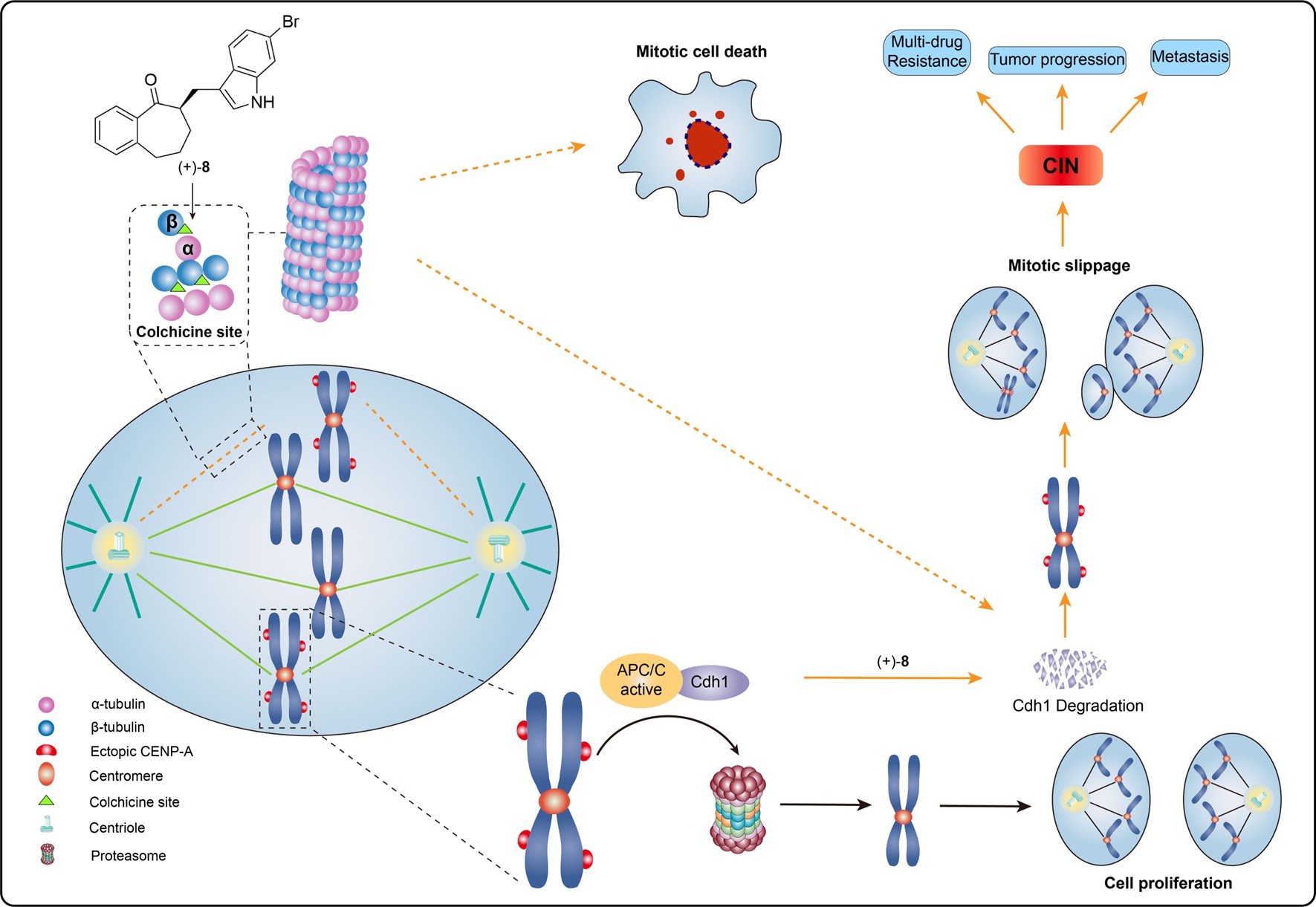
71. Yan Peng, Yumeng Zhang, Ruan Fang, Hao Jiang, Gongcai Lan, Zhou Xu, Yajie Liu, Zhaoyang Nie, Lu Ren, Fengcan Wang, Shou-De Zhang, Yuyong Ma, Peng Yang, Hong-Hua Ge, Wei-Dong Zhang,* Cheng Luo,* Ang Li,* and Weiwei He,* Target identification and mechanistic characterization of indole terpenoid mimics: proper spindle microtubule assembly is essential for Cdh1-mediated proteolysis of CENP-A, Adv. Sci. 2024, 11, 2305593 (Y. P., Y. Z., R. F. and H. J. contributed equally).
70. Qun Wang, Jinxin Wang, Dianping Yu , Qing Zhang, Hongmei Hu, Mengting Xu, Hongwei Zhang, Saisai Tian, Guangyong Zheng, Dong Lu, Jiajia Hu, Mengmeng Guo, Minchen Cai, Xiangxin Geng, Yanyan Zhang, Jianhua Xia, Xing Zhang, Ang Li, Sanhong Liu,* and Weidong Zhang,* Benzosceptrin C induces lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting DHHC3, Cell Rep. Med. 2024, 5, 101357 (Q. W., J. W., D. Y. and Q. Z. contributed equally).
69. Ruonan Wang, Hao Xu, Arpan Banerjee, Zhongwen Cui, Yuyong Ma, William G. Whittingham, Peng Yang,* and Ang Li,* Mild approach to nucleoside analogues via photoredox/Cu-catalyzed decarboxylative C-N bond formation. Total synthesis of oxetanocin A, Org. Lett. 2024, 26, 2691–2696 (R. W., H. X. and A. B. contributed equally).
68. Wenhao Zhang, Ming Lu, Lu Ren, Xiang Zhang, Shaonan Liu, Mengyu Ba, Peng Yang, and Ang Li,* Total synthesis of four classes of Daphniphyllum alkaloids, J. Am. Chem. Soc. 2023, 145, 26569–26579 (M. L. and L. R. contributed equally).
67. Fan Yang, Jie Zhang, Jiacheng Li, Wenbo Ye, Ang Li,* and Weiwei He,* Synthesis of a glucose conjugate of pristimerin and evaluation of its anticancer activity, Chin. Chem. Lett. 2023, 34, 107438.
66. S. Liu, J. Wang, Y. Ma, X. Cao, W. Zhang,* and A. Li,* Construction of alkyl-substituted 7-norbornenones through Diels−Alder cycloaddition of electron-deficient olefins and a cyclopentadienone derivative generated in situ, Chin. Chem. Lett. 2022, 33, 2041–2043.
65. Zhuo Wang, Yiren Xiao, Song Wu, Jianghua Chen, Ang Li, and Evangelos C. Tatsis,* Deciphering and reprogramming the cyclization regioselectivity in bifurcation of indole alkaloid biosynthesis, Chem. Sci. 2022, 13, 12389–12395 (Z. W. and Y. X. contributed equally).
64. Bing-Chao Yan, Min Zhou, Jian Li, Xiao-Nian Li, Shi-Jun He, Jian-Ping Zuo, Han-Dong Sun, Ang Li,* and Pema-Tenzin Puno,* (–)-Isoscopariusin A, a Naturally Occurring Immunosuppressive Meroditerpenoid: Structure Elucidation and Scalable Chemical Synthesis, Angew. Chem. Int. Ed. 2021, 60, 12859–12867 (B. Y. and M. Z. contributed equally).
63. Jiacheng Li, Yuyong Ma, Xiang Zhang, Xin Cao, Hegui Gong,* and Ang Li,* Expeditious and scalable preparation of a Li−Thiele reagent for amine-based bioconjugation, Chin. Chem. Lett. 2021, 32, 700–702.
62. Peng Yang, Jian Li, Li Sun, Ming Yao, Xiang Zhang, Wei-Lie Xiao, JianHua Wang, Ping Tian, Han-Dong Sun, Pema-Tenzin Puno, and Ang Li,* Elucidation of the structure of pseudorubriflordilactone B by chemical synthesis, J. Am. Chem. Soc. 2020, 142, 13701–13708.
61. Shupeng Zhou, Kaifu Xia, Xuebing Leng, and Ang Li,* Asymmetric total synthesis of arcutinidine, arcutinine, and arcutine, J. Am. Chem. Soc. 2019, 141, 13718–13723.
60. Alán Aspuru-Guzik, Mu-Hyun Baik, Shankar Balasubramanian, Rahul Banerjee, Suzanne Bart, Nadine Borduas-Dedekind, Sukbok Chang, Peng Chen, Clemence Corminboeuf, François-Xavier Coudert, Leroy Cronin, Cathleen Crudden, Tanja Cu, Abigail G. Doyle, Chunhai Fan, Xinliang Feng, Danna Freedman, Shuhei Furukawa, Suhrit Ghosh, Frank Glorius, Malika Jefries-EL, Nathalie Katsonis, Ang Li, Sara Snogerup Linse, Silvia Marchesan, Nuno Maulide, Anat Milo, Alison R. H. Narayan, Pance Naumov, Cristina Nevado, Tebello Nyokong, Rosa Palacin, Marc Reid, Carol Robinson, Gregory Robinson, Richmond Sarpong, Corinna Schindler, Gabriela S. Schlau-Cohen, Timothy W. Schmidt, Roberta Sessoli, Yang Shao-Horn, Hanadi Sleiman, John Sutherland, Annette Taylor, Akif Tezcan, Mariola Tortosa, Aron Walsh, Allan J. B. Watson, Bert M. Weckhuysen, Emily Weiss, Daniela Wilson, Vivian W.-W. Yam, Xueming Yang, Jackie Y. Ying, Tehshik Yoon, Shu-Li You, Aldo J. G. Zarbin and Hua Zhang, Charting a course for chemistry, Nature Chem. 2019, 11, 286–294. 59. Xiang Zhang, Badrinath N. Kakde, Rui Guo, Sonyabapu Yadav, Yucheng Gu, and Ang Li,* Total synthesis of echitamine, akuammiline, rhazicine, and pseudoakuammigine, Angew. Chem. Int. Ed. 2019, 58, 6053–6058 (X. Z. and B. N. K. contributed equally).
58. Gongcai Lan, Jie Zhang, Wenbo Ye, Fan Yang, Ang Li,* Weiwei He,* and Wei-Dong Zhang,* Celastrol as a tool for the study of the biological events of metabolic diseases, Sci. China Chem. 2019, 62, 409–416.
57. Yu Chen, Lianchao Liu, Dimin Wu, Yu-Peng He,* and Ang Li,* A one-pot protocol for copper-mediated azide–alkyne cycloaddition using alkenyl triflate precursors, Chin. Chem. Lett. 2019, 30, 269–270 (Y. C. and L. L. contributed equally).
56. Stephanie R. Hare, Ang Li, and Dean J. Tantillo,* Post-transition state bifurcations induce dynamical detours in Pummerer-like reactions, Chem. Sci. 2018, 9, 8937–8945.
55. Wenbo Ye and Ang Li,* A practical route to 3D molecular diversity, Nature 2018, 560, 314–316.
54. Zhaohong Lu, Xiang Zhang, Zhicong Guo, Yu Chen, Tong Mu, and Ang Li,* Total synthesis of aplysiasecosterol A, J. Am. Chem. Soc. 2018, 140, 9211–9218 (Z. L., X. Z., and Z. G. contributed equally).
53. Shupeng Zhou, Rui Guo, Peng Yang, and Ang Li,* Total synthesis of septedine and 7-deoxyseptedine, J. Am. Chem. Soc. 2018, 140, 9025–9029 (S. Z. and R. G. contributed equally).
52. Wenhao Zhang, Ming Ding, Jian Li, Zhicong Guo, Ming Lu, Yu Chen, Lianchao Liu, Yun-Heng Shen, and Ang Li,* Total synthesis of hybridaphniphylline B, J. Am. Chem. Soc. 2018, 140, 4227–4231 (W. Z., M. D., and J. L. contributed equally).
51. Yu Chen, Wenhao Zhang, Lu Ren, Jian Li, and Ang Li,* Total syntheses of daphenylline, daphnipaxianine A, and himalenine D, Angew. Chem. Int. Ed. 2018, 57, 952–956 (Y. C. and W. Z. contributed equally).
50. Jian Li, Wenhao Zhang, Fei Zhang, Yu Chen, and Ang Li,* Total synthesis of daphniyunnine C (longeracinphyllin A), J. Am. Chem. Soc. 2017, 139, 14893–14896 (J. L. and W. Z. contributed equally).
49. Yong Li, Jian Li, Hanfeng Ding,* and Ang Li,* Recent advances on the total synthesis of alkaloids in mainland China, National Science Review 2017, 4, 397–425 (Y. L. and J. L. contributed equally).
48. Zhongyin Zhang, Jinxin Wang, Jian Li, Fan Yang, Guodu Liu, Wenjun Tang, Weiwei He, Jian-Jun Fu, Yun-Heng Shen,* Ang Li,* and Wei-Dong Zhang,* Total synthesis and stereochemical assignment of delavatine A: Rh-catalyzed asymmetric hydrogenation of indene-type tetrasubstituted olefins and kinetic resolution through Pd-catalyzed triflamide-directed C−H olefination, J. Am. Chem. Soc. 2017, 139, 5558–5567 (Z. Z., J. W., and J. L. contributed equally).
47. Qingbo Zhang, Huixian Li, Lu Yu, Yu Sun, Yiguang Zhu, Hanning Zhu, Liping Zhang, Shu-Ming Li, Yuemao Shen, Changlin Tian, Ang Li, Hung-wen Liu,* and Changsheng Zhang,* Characterization of the flavoenzyme XiaK as an N-hydroxylase and implications in indolosesquiterpene diversification, Chem. Sci .2017, 8, 5067–5077 (Q. Z. and H. L. contributed equally).
46. Wenhao Zhang and Ang Li,* A radical step forward, Nature Chem. 2017, 9, 198–199.
45. Hailong Li, Qifeng Chen, Zhaohong Lu, and Ang Li,* Total syntheses of aflavazole and 14-hydroxyaflavinine, J. Am. Chem. Soc. 2016, 138, 15555–15558 (H. L. and Q. C. contributed equally). 44. Q.-Y. Zheng* and A. Li,* The bloom of natural product chemistry in China, Sci. China Chem. 2016, 59, 1059–1060.
43. Jinpeng Pei, Shupeng Zhou, Fan Yang, Yu Sun, Ang Li,* Wei-Dong Zhang,* and Weiwei He,* Identification and mechanistic studies of a cell cycle regulator JP18 from a library of synthetic indole terpenoid mimics, Chem. Asian J. 2016, 11, 2715–2718.
42. Peng Yang, Ming Yao, Jian Li, Yong Li, and Ang Li,* Total synthesis of rubriflordilactone B, Angew. Chem. Int. Ed. 2016, 55, 6964–6968 (P. Y. and M. Y. contributed equally).
41. Xiaowen Yang, Dimin Wu, Zhaohong Lu, Hongbin Sun, and Ang Li,* A mild preparation of alkynes from alkenyl triflates, Org. Biomol. Chem. 2016, 14, 5591–5594 (X. Y. and D. W. contributed equally).
40. Yong Li, Shugao Zhu, Jian Li, and Ang Li,* Asymmetric total syntheses of aspidodasycarpine, lonicerine, and the proposed structure of lanciferine, J. Am. Chem. Soc. 2016, 138, 3982–3985 (Y. L. and S. Z. contributed equally).
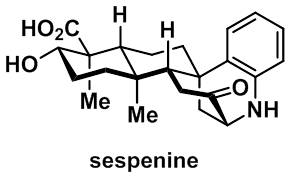
39. Yu Sun, Zhanchao Meng, Pengxi Chen, Deliang Zhang, Martin Baunach, Christian Hertweck, and Ang Li,* A concise total synthesis of sespenine, a structurally unusual indole terpenoid from Streptomyces, Org. Chem. Front. 2016, 3, 368–374 (Y. S. and Z. M. contributed equally).
38. Ming Yang, Xiaowen Yang, Hongbin Sun, and Ang Li,* Total synthesis of ileabethoxazole, pseudopteroxazole, and seco-pseudopteroxazole, Angew. Chem. Int. Ed. 2016, 55, 2851–2855.
37. Zhaohong Lu, Hailong Li, Ming Bian, and Ang Li,* Total synthesis of epoxyeujindole A, J. Am. Chem. Soc. 2015, 137, 13764–13767. (Z. L. and H. L. contributed equally).
36. Shupeng Zhou, Hao Chen, Yijie Luo, Wenhao Zhang, and Ang Li,* Asymmetric total synthesis of mycoleptodiscin A, Angew. Chem. Int. Ed. 2015, 54, 6878–6882 (S. Z. and H. C. contributed equally).
35. Ming Yang, Jian Li, and Ang Li,* Total synthesis of clostrubin, Nat. Commun. 2015, 6, 6445.
34. Ming Wan, Ming Yao, Junyu Gong, Peng Yang, Hua Liu,* and Ang Li,* Synthesis of the tetracyclic core of chlorospermines,
33. Huixian Li, Yu Sun, Qingbo Zhang, Yiguang Zhu, Shu-Ming Li, Ang Li, and Changsheng Zhang,* 32. Zhanchao Meng, Haixin Yu, Li Li, Wanyin Tao, Hao Chen, Ming Wan, Peng Yang, David J. Edmonds, Jin Zhong, and Ang Li,* Total synthesis and antiviral activity of indolosesquiterpenoids from the xiamycin and oridamycin families, Nature Communications 2015, 6, 6096.
31. Xiaochun Xiong, Deliang Zhang, Jian Li, Yu Sun, Shupeng Zhou, Ming Yang, Huawu Shao,* and Ang Li,* Synthesis of indole terpenoid mimics via a functionality-tolerated Eu(fod)3-catalyzed conjugate addition, Chem. Asian J. 2015, 10, 869–872 (X. X. and D. Z. contributed equally).
30. M. Isobe,* A. Nishida,* Y.-M. Choo, N. A. Rahman,* P. Ploypradith, S. Ruchirawat,* G.-Q. Lin, A. Li,* Z.-J. Yao,* B.-J. Uang,* C.-C. Liao, P. Chiu,* B. M. Kim,* and T. P. Loh,* The last and next decades of the Asian Core Program on Cutting-Edge Organic Chemistry in Asia, Chem. Asian J. 2015, 10, 790–804.
29. Jian Li, Peng Yang, Ming Yao, Jun Deng, and Ang Li,* Total synthesis of rubriflordilactone A, J. Am. Chem. Soc. 2014, 136, 16477–16480 (J. L. and P. Y. contributed equally).
28. Zhaohong Lu, Ming Yang, Pengxi Chen, Xiaochun Xiong, and Ang Li,* Total synthesis of hapalindole-type natural products, Angew. Chem. Int. Ed. 2014, 53, 13840–13844 (Z. L. and M. Y. contributed equally).
27. Shupeng Zhou, Deliang Zhang, Yu Sun, Ruofan Li, Wenhao Zhang, and Ang Li,* Intermolecular conjugate addition of pyrroloindoline and furoindoline radicals to α, β-unsaturated enones via photoredox catalysis, Adv. Synth. Catal. 2014, 356, 2867–2872 (S. Z. and D. Z. contributed equally).
26. Yu Sun, Pengxi Chen, Deliang Zhang, Martin Baunach, Christian Hertweck, and Ang Li,* Bioinspired total synthesis of sespenine, Angew. Chem. Int. Ed. 2014, 53, 9012–9016 (Y. S. and P. C. contributed equally).
25. Jun Deng, Shupeng Zhou, Wenhao Zhang, Jian Li, Ruofan Li, and Ang Li,* Total synthesis of taiwaniadducts B, C, and D, J. Am. Chem. Soc. 2014, 136, 8185–8188 (J. D. and S. Z. contributed equally).
24. Chunyun Wan, Jun Deng, Hua Liu,* Ming Bian,* and Ang Li,* Recent advances of intermolecular Diels–Alder reaction in bio-inspired synthesis of natural products, Sci China Chem. 2014, 57, 926–929 (C. W. and J. D. contributed equally). 23. Xiaochun Xiong, Yong Li, Zhaoyong Lu, Ming Wan, Jun Deng, Shuhang Wu, Huawu Shao,* and Ang Li,* Synthesis of the 6,6,5,7-tetracyclic core of daphnilongeranin B, Chem. Commun. 2014, 50, 5294–5297 [(X. X. and Y. L. contributed equally). This article is part of themed collection: 2014 Emerging Investigators.].
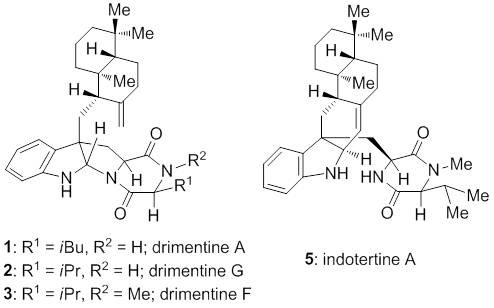
22. Haixin Yu, Chunyun Wan, Jing Han,* and Ang Li,* A protocol for α-bromination of β-substituted enones, Acta Chim. Sinica 2013, 71, 1488–1491 (H. Y. and C. W. contributed equally). 21. Yu Sun, Ruofan Li, Wenhao Zhang, and Ang Li,* Total synthesis of indotertine A and drimentines A, F, and G, Angew. Chem. Int. Ed. 2013, 52, 9201–9204 (Y. S. and R. L. contributed equally).
20. Zhaoyong Lu, Yong Li, Jun Deng, and Ang Li,* Total synthesis of the Daphniphyllum alkaloid daphenylline, Nature Chemistry 2013, 5, 679–684 (Z. L. and Y. L. contributed equally).
19. Jun Deng, Ruofan Li, Yijie Luo, Jian Li, Shupeng Zhou, Yongjian Li, Jingyu Hu, and Ang Li,* Divergent total synthesis of taiwaniaquinones A and F and taiwaniaquinols B and D, Org. Lett. 2013, 15, 2022–2025 (J. D. and R. L. contributed equally). 
18. Sen Li, Jing Han,* and A. Li,* Interrupted Fisher indole synthesis and its applications to alkaloid synthesis , Acta Chim. Sinica 2013, 71, 295–298 (highlight). 17. Ming Bian, Zhen Wang, Xiaochun Xiong, Yu Sun, Carlo Matera, K. C. Nicolaou,* and Ang Li,* Total syntheses of anominine and tubingensin A, J. Am. Chem. Soc. 2012, 134, 8078–8081 [M. B. and Z. W. contributed equally; JACS Most Read Articles in 12 months (08/2011-07/2012)]. 
16. Jun Deng, Bo Zhu, Zhaoyong Lu, Haixin Yu, and Ang Li,* Total synthesis of (–)-fusarisetin A and reassignment of the absolute configuration of its natural counterpart, J. Am. Chem. Soc. 2012, 134, 920–923 [J. D. and B. Z. contributed equally; JACS Most Read Articles in 12 months (05/2011-04/2012)].

15. C.-C. Tseng, H. Ding, A. Li, Y. Guan, D. Y.-K. Chen, A Modular Synthesis of Salvileucalin B Structural Domains, Org. Lett. 2011, 1, 4410–4413.
14. K. C. Nicolaou, A. Li, D. J. Edmonds, G. S. Tria, S. P. Ellery, Total Syntheses of Platensimycin and
Related Natural Products, J. Am. Chem.
Soc. 2009, 131, 16905–16918.
13. K. C. Nicolaou, A. Li, S. P. Ellery, D. J.
Edmonds, Rhodium-Catalyzed Asymmetric Enyne Cycloisomerization of Terminal Alkynes and Formal Total Synthesis of (–)-Platensimycin, Angew. Chem. Int. Ed. 2009,
48, 6293–6295.
12. K. C. Nicolaou, A. F. Stepan, T. Lister, A.
Li, A. Montero, G. S. Tria, C. I. Turner, Y.
Tang, J. Wang, R. M. Denton, D. J. Edmonds, Design, Synthesis and Biological
Evaluation of Platensimycin Analogs with Varying Degrees of Molecular
Complexity, J. Am. Chem. Soc. 2008, 130, 13110–13119.
11. K. C. Nicolaou, A. Li, Total Syntheses and Structural
Revision of α- and β-Diversonolic Esters and Total
Syntheses of Diversonol and Blennolide C, Angew. Chem. Int. Ed. 2008, 47, 6579–6582.
10. K. C. Nicolaou, Y.
Tang, J. Wang, A. F. Stepan, A. Li, A. Montero, Total Synthesis and Antibacterial Propoties of Carbaplatensimycin,
J. Am. Chem. Soc. 2007, 129, 14850–14851.
9. K. C. Nicolaou,
D. J. Edmonds, A. Li, G. S. Tria, Asymmetric Total Syntheses of Platensimycin,
Angew. Chem. Int. Ed. 2007, 46, 3942–3945.
8. K. C. Nicolaou,
A. Li. D. J. Edmonds, Total
Synthesis of Platensimycin, Angew. Chem. Int. Ed. 2006,
45, 7086–7090. This work was highlighted
by Nature 2006, 443, 726, Chemical and Engineering News 2006, 84(41), 12, and Chemistry
World 2006, 3(11), 21.
7. K. C. Nicolaou,
R. M. Denton, A. Lenzen, D. J. Edmonds, A. Li, R. M. Milburn, S. T. Harrison, Stereocontrolled Synthesis of Model Core Systems of Lomaiviticins A and B, Angew. Chem. Int. Ed. 2006,
45, 2076–2081.
6. B. Liang, J. Liu, Y.-X. Gao, K. Wongkhan, D.-X. Shu, Y. Lan, A. Li, A. S. Batsanov,
J. A. H. Howard, T. B. Marder, J.-H. Chen, Z. Yang, Synthesis of Thiourea-Oxazolines, a New Class
of Chiral
S,N-Heterobidentate Ligands:
Application in Pd-Catalyzed Asymmetric
Bis(methoxycarbonylation)
of Terminal Olefins, Organometallics 2007, 26, 4756–4762.
5. Z. Xiong, N. Wang, M. Dai, A. Li, J. Chen, Z. Yang, Synthesis of Novel Palladacycles and Their Application in Heck and Suzuki Reactions under Aerobic Conditions, Org. Lett. 2004, 6, 3337–3340.
4. Y. Zhang, A. Li, Z. Yan, G. Xu, C. Liao, C. Yan, (ZrO2)0.85(REO1.5)0.15
(RE = Sc, Y) solid solutions prepared via three Pechini-type
gel routes: 1. gel formation and calcination
behaviors, Journal of Solid State
Chemistry 2003, 171, 434–438.
3. Y. Zhang, A. Li, Z. Yan, G. Xu, C. Liao, C. Yan, (ZrO2)0.85(REO1.5)0.15
(RE=Sc, Y) solid solutions prepared via three Pechini-type
gel routes: 2-sintering and electrical properties, Journal of Solid State Chemistry 2003, 171, 439–443.
2. Y. Zhang, A. Li, Z. Yan, C. Liao, C. Yan, Calcination
Time Effects on the Particle Size , Specific Surface Area and Morphology of
Rare Earth Oxides (III), Journal
of the Chinese Rare Earth Society (Chinese
Edition) 2002, 20, 170–172.
1. Y. Zhang, Z. Yan, A. Li, X, Jiang, L. Gu, C. Liao, C.
Yan, Effects of Precipitation Conditions on Specific Surface Area and
Morphology of Rare Earth Oxides(II), Journal
of the Chinese Rare Earth Society (Chinese
Edition) 2001, 19, 471–473.
|
|
|
Copyright @ 2013 The Ang Li Group, Shanghai Institute of Organic Chemistry, CAS |
|